North Carolina State University (PETRO)
ENGINEERING THE OPTIMIZED BIOFUEL CROP
UPDATED: JANUARY 10, 2017
PROJECT TITLE: Jet Fuel From Camelina Sativa: A Systems Approach | Developing a Dedicated High-Value Biofuels Crop
PROGRAM: Plants Engineered to Replace Oil (PETRO)
AWARD: $8,556,904 | $3,751,152
PROJECT TEAM: North Carolina State University (Lead); Metabolix | University of Massachusetts (Lead); Washington State University; Metabolix
PROJECT TERM: January 2012 – December 2016 | January 2012 – December 2015
PRINCIPAL INVESTIGATOR (PI): Dr. Heike Sederoff | Dr. Danny Schnell
TECHNICAL CHALLENGE
Biofuels offer renewable alternatives to petroleum-based fuels that can reduce net greenhouse gas (GHG) emissions dramatically. Today’s vision for cost competitive domestically produced biofuels must address challenges in sustainable production of bio-energy crops, and cost-effective production of drop-in fuels for demanding applications such as air transportation. Sustainability requires crops with high yields per land area, the ability to use land that is less useful for food crops, and reduced agronomic inputs (e.g. water, fertilizer, etc.). Cost-effective production of drop-in fuels requires biomass products that need limited processing, for which oil-rich crops are promising. However, existing oil-rich crops do not provide enough energy yield per land area to be a cost-effective alternative for production of transportation fuel at scale. Traditional breeding approaches in the major row crops produce between 1-2% increases in yield annually, which is insufficient to generate the yields needed for economically viable drop-in fuels.
TECHNICAL OPPORTUNITY
Recent advances in synthetic biology and decreased cost of custom DNA synthesis now allow complex metabolic pathway engineering in plants. These technologies are enabling researchers to incorporate novel biosynthetic pathways into both model plants and row crops for the field. Camelina sativa is a promising oilseed crop that can grow in geographies that traditional row crops do not, has a short generation time, and requires low agronomic inputs. A rapid and efficient genetic transformation method was previously developed for Camelina which allows researchers to engineer complex traits into the plant.
INNOVATION DEMONSTRATION
Under the Plants Engineered To Replace Oil (PETRO) program, ARPA-E funded projects at North Carolina State University (NCSU) and the University of Massachusetts (UMass) focused on engineering Camelina to dramatically increase the oil yield of the crop, and the composition of the oil to be a better fuel. The two projects had similar goals, but used different biological approaches. UMass teamed with Washington State University (WSU) and Metabolix Inc., a biotechnology company that is a pioneer in the field of metabolic engineering. NCSU added Metabolix as a commercial partner in 2015. As their projects matured, the two teams developed a collaboration to combine the synergistic approaches from each of their projects.
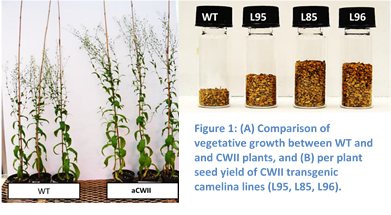
Figure 1: (A) Comparison of vegetative growth between WT and CWII plants, and (B) per plant seed yield of CWII transgenic camelina lines (L95, L85, L96).
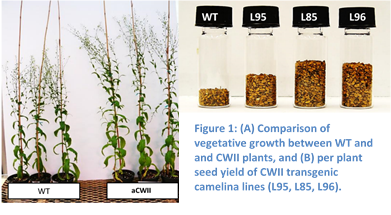
Figure 2: (A) Increased photosynthetic activity, (B) enhanced resistance to water stress, and (C) increased oil yields in Camelina transporter lines.
NCSU Project
The NCSU team’s goal was to double the seed yield of the crop through increased photosynthetic efficiency and channeling the energy captured through photosynthesis to oil production. NCSU identified numerous novel plant genes and metabolic pathways to increase carbon dioxide (CO2) fixation, reduce photorespiratory energy and carbon loss, increase yield, and shorten the growth cycle of Camelina. The lines engineered with these traits that showed increased biomass and seed yields over multiple generations in the greenhouse were seleacted for further characterization and development. One of the most promising targets are cell wall invertase inhibitors (CWIIs), which regulate the transport of sugar from the photosynthetically active tissues (like leaves) in the plant to the non-green roots, flowers and seeds. NCSU engineered this target gene to increase the levels of sugar available for biomass production and seed yield, which led to Camelina with higher vegetative biomass (>20%), higher rates of photosynthesis and increased seed yield (40-80%) compared to wild type (WT) control (Figure 1). Another pathway reduces the loss of CO2 from photorespiration during photosynthesis. Camelina plants expressing enzymes for this pathway flower and set seed almost a week earlier than wild type plants without reducing yield. This is valuable to farmers because it allows them greater flexibility with sowing and harvesting. Flowering though, is very dependent on environmental conditions, so this phenotype requires testing under field conditions.
UMass Project
The UMass team used alternative strategies to increase photosynthetic activity and seed yield. Primarily, UMass worked to introduce carbon concentrating mechanisms into the plant’s cells to increase the intracellular levels of CO2, and increase photosynthesis. UMass evaluated a number of metabolite transporters from microalgae and cyanobacteria in Camelina, and identified one that reliably increased biomass and seed yield in Camelina over multiple generations. The transporter functioned by increasing the availability of CO2 within the cell to significantly increase the catalytic rate of CO2 fixation by RuBisCO, and increased photosynthetic efficiency in the Camelina plants 10-25% (Figure 2). In an initial small-scale field trial, Camelina lines expressing the transporter produced up to 75% more seed and increased oil yields than wild type plants (Figure 2).
The Camelina plants expressing components of the carbon concentrating mechanism also showed enhanced resistance to water stress (Figure 2), due to the fact that the plants more efficiently fix CO2 and can reduce gas exchange between leaves and the atmosphere.
Collaborative Work
As the UMass metabolite transporter functioned through a distinct mechanism from NCSU’s traits, ARPA-E encouraged the two teams to work together. NCSU and UMass stacked the transporter and cell wall invertase inhibitor together in Camelina and observed in the laboratory an additional 15% increase in vegetative biomass production and increased seed yield in the stacked lines compared to the parent line. Metabolix partnered with NCSU in 2015 to test promising Camelina lines from the NCSU and UMass programs in a field trial as a first step to incorporating the lines or traits into Metabolix’s commercial pipeline. Due to Camelina’s short lifecycle, homozygous seeds ready for field trials can be obtained within a year allowing for rapid field testing of promising traits. Field trials were established in multiple locations in 2016, and are now underway to test the most promising Camelina traits from these two PETRO projects against Metabolix’s existing high yielding Camelina varieties. At the end of the project period, the field data on the oil yields from the Camelina lines expressing the PETRO traits will be independently validated by Oak Ridge National Laboratory.
PATHWAY TO ECONOMIC IMPACT
The key for the successful adoption of a new crop like Camelina is grower acceptance. NCSU produced economic models under ARPA-E support that show that increases in Camelina seed oil yield of 70%, which is the level of increase NCSU observed in the greenhouse with its traits, would more than triple farmers’ profits. Figure 3 shows data for 67% and 133% increases in seed yield per acre as compared with the average of 2009-2013 profits from corn in the eastern and western corn belt. Although, in some regions Camelina yields are not sufficient to provide enough economic incentive to displace corn and soy acreage, Camelina can be grown in regions that are not well suited for these traditional crops. Following the field tests, NCSU will incorporate the new data into its technoeconomic model to evaluate the economic potential as a biofuel feedstock.
ECONOMIC DEVELOPMENT
The pathway to economic impact for this project will be determined by the outcomes of the field trials in 2016, for which Metabolix has licensed the promising PETRO traits from UMass and NCSU. The successful traits will be moved into Metabolix’s commercial Camelina varieties for further large-scale field trials. Continuing work to further improve Camelina yield will be supported in part by a $2.5 million award to Metabolix and NCSU from the DOE BioEnergy Technology Office, covering activities to further develop Camelina lines with increased seed yield and oil content using a genome editing approach.
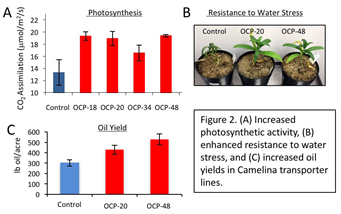
Figure 3: Modeled farmer profit with increasing Camelina yields (green bars) vs. 5 year average profits from high productivity “corn only” farmland in the Eastern and Western corn belt. Data sources: NC State economic model for Camelina; University of Illinois and Purdue University for 2009-2013 corn profits.
In addition to the Camelina work, using internal funding, Metabolix is also currently working to transfer select licensed traits into C3 row crops such as rice, canola and soybeans, while evaluating whether any of the traits will also increase yield in C4 crops, which include major row crops such as corn, sorghum, or sugar cane. The results of this analysis will guide future commercialization efforts and licensing/negotiations with major agricultural companies.
Metabolix will develop first markets for the NCSU/UMass traits in high-value feedstock market opportunities in the food, feed and fuel sectors. These select market segments will support the development expense of new plant feedstocks, facilitating their staged introduction into commodity markets.
LONG-TERM IMPACTS
Success in the first-market strategy outlined above is expected to provide a foundation for future commercialization efforts in biofuels and advanced biofuels. Camelina derived jet fuel has been successfully tested by the U.S. Air Force, Navy, and by commercial airlines. The PETRO technology has the potential to make this approach economically competitive.
Furthermore, if the demonstrated large increases in yield do readily transfer into C3 and C4 row crops, this technology could make a major contribution towards increasing world food production while minimizing impacts on water, energy, and land resources.
INTELLECTUAL PROPERTY AND PUBLICATIONS
The NCSU team had 18 inventions and filed 4 patent applications, the UMass team had 10 inventions, and the combined NCSU and UMass team had 1 joint invention, for a total of 29 inventions and 4 patent applications.
The NCSU and UMass teams have published the scientific underpinnings of these technologies in the open literature. A list of scientific publications and patent applications is provided below:
NCSU
“Technoeconomic analysis of jet fuel production from hydrolysis, decarboxylation, and reforming of camelina oil,” R. Natelson, W. Wang, W. Roberts, K. Zering, Biomass & Bioenergy, Vol. 75, pg. 23-34, DOI: 10.1016/j.biombioe.2015.02.001, 2015.
"A photorespiratory bypass increases plant growth and seed yield in biofuel crop Camelina sativa." J. Dalal., H. Lopez, N. Vasani, Z. Hu, J. Swift, R. Yalamanchili, H. Sederoff, Biotechnology for biofuels 8.1, p. 1, 2015.
“Tissue-specific production of limonene in Camelina sativa with the Arabidopsis promoters of genes BANYULS and FRUITFULL,” M. Borghi, D. Xie, Planta, 1;243(2):549-61, 2016
“Overexpression of a synthetic insect–plant geranyl pyrophosphate synthase gene in Camelina sativa alters plant growth and terpene biosynthesis.” J. Xi, L. Rossi, X. Lin, D. Xie. Planta. 2016 Mar 29:1-6.
“A novel gateway-compatible binary vector series (PC-GW) for flexible cloning of multiple genes for genetic transformation of plants.” J. Dalal, R. Yalamanchili, C. La Hovary, M. Ji, M. Rodriguez-Welsh, D. Aslett, S. Ganapathy, A. Grunden, H. Sederoff, R. Qu, Plasmid. 2015 Sep 30;81:55-62.
UMass
“Transcriptome profiling of Camelina sativa to identify genes involved in triacylglycerol biosynthesis and accumulation in the developing seeds,” H. Abdullah, P. Akbari, B. Paulose, D. Schnell, W. Qi, Y. Park, A. Pareek, O. Dhankher, Biotechnol Biofuels. 9:136. doi: 10.1186/s13068-016-0555-5, 2016.
“Genome and Transcriptome of Clostridium phytofermentans, Catalyst for the Direct Conversion of Plant Feedstocks to Fuels,” M. Coppi, J. Hayes, A. Tolonen, T. Warnick, W. Latouf, D. Amisano, A. Biddle, S. Mukherjee, N. Ivanova, A. Lykidis, M. Land, L. Hauser, N. Kyrpides, B. Henrissat, J. Lau, D. Schnell, G. Church, S. Leschine, J. Blanchard, PLoS One 10(6):e0118285. doi: 10.1371/journal.pone.0118285, 2015.
“Involvement of a Bacterial Microcompartment in the Metabolism of Fucose and Rhamnose by Clostridium phytofermentans,” E. Petit, W. LaTouf, M. Coppi, T. Warnick, D. Currie, et al., PLoS ONE 8(1): e54337. doi:10.1371/journal.pone.0054337, 2013.
“Biological conversion assay using Clostridium phytofermentans to estimate plant feedstock quality,” S. Lee, T. Warnick, S. Pattathil, J. Alvelo-Maurosa, M. Serapiglia, H. McCormick, V. Brown, N. Young, D. Schnell, L. Smart, M. Hahn, J. Pedersen, S. Leschine, S. Hazen, Biotechnology for Biofuels 5:5, 2012.
